Press Release
Akebia Presents Results from its PRO₂TECT Global Phase 3 Program of Vadadustat for the Treatment of Anemia due to Chronic Kidney Disease in Adult Patients Not on Dialysis During Late-Breaking Session at American Society of Nephrology Kidney Week 2020 Reimagined
- As previously announced, PRO2TECT data show vadadustat achieved primary and key secondary efficacy endpoints in each study, but did not meet the primary safety endpoint
- Newly presented pre-specified regional analyses show vadadustat demonstrated no clinically meaningful increase in risk of MACE, expanded MACE and all-cause mortality compared to an active comparator in U.S. patients treated to a target hemoglobin range of 10 to 11 g/dL, consistent with U.S. treatment guidelines
- Investor briefing webcast today at 4:10 p.m. ET
"The PRO2TECT results show that orally administered vadadustat achieved both primary and secondary efficacy endpoints in patients not on dialysis with anemia associated with CKD. The newly presented analyses showed that there were regional differences with respect to MACE, expanded MACE and all-cause mortality, consistent with well-known, differing regional hemoglobin treatment target guidelines," said
The PRO2TECT data are being presented today during a late-breaking oral presentation, titled "Global Phase 3 Clinical Trials of Vadadustat vs Darbepoetin Alfa for Treatment of Anemia in Patients with Non-Dialysis-Dependent Chronic Kidney Disease" (Abstract FR-OR54) at ASN Kidney Week. Akebia's vadadustat development program also includes INNO2VATE, the global Phase 3 program for the treatment of anemia due to CKD in adult patients on dialysis. Results from this program were presented at ASN Kidney Week in an oral presentation on
Highlights of the PRO2TECT ASN Kidney Week Presentation:
Efficacy:
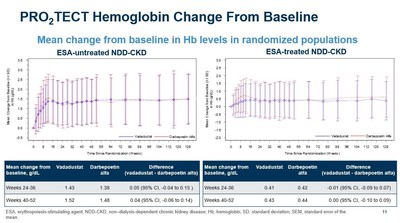
- As previously reported, vadadustat achieved the primary and key secondary efficacy endpoints in each of the two PRO2TECT studies, which demonstrated non-inferiority of vadadustat to darbepoetin alfa as measured by a mean change in Hb between baseline and the primary evaluation period (weeks 24 to 36) and secondary evaluation period (weeks 40 to 52). Non-inferiority was achieved as the lower bound of the 95% confidence interval for the between-group difference of the mean Hb change did not fall below the pre-specified non-inferiority margin (-0.75 g/dL).
Safety:
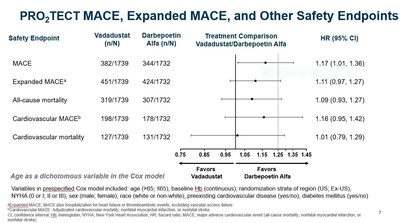
- As previously reported, vadadustat did not meet the primary safety endpoint of the PRO2TECT program defined as non-inferiority of vadadustat versus darbepoetin alfa in time to first occurrence of major adverse cardiovascular events (MACE), which is the composite of all-cause mortality, non-fatal myocardial infarction (MI), or non-fatal stroke across both PRO2TECT studies.
- The analysis of the primary safety endpoint used a Cox regression model that was pre-specified to be adjusted for certain variables, including age dichotomized >65 and ≤65 years.
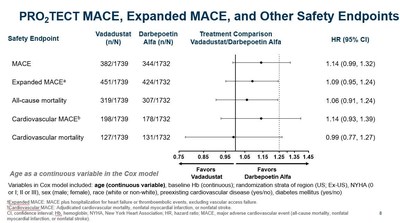
- In order to optimize the Cox model used to analyze the primary safety endpoint and increase its statistical power in regional subsets, age was rescaled from dichotomous to a continuous variable.
- In the optimized Cox model, the hazard ratios (HR) were attenuated and the confidence intervals (CI) were narrowed.
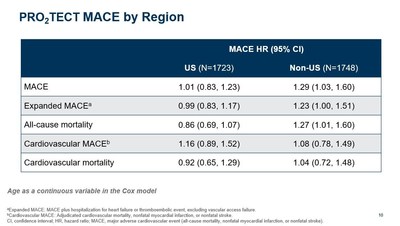
- The PRO2TECT study analysis plan was prospectively designed to analyze the effect of regional differences, most notably, well-known differences in Hb treatment targets. Within the study,
U.S. patients were treated to a target Hb range of 10 to 11 g/dL and non-U.S. patients were treated to a target Hb range of 10 to 12 g/dL. - In a pre-specified regional analysis using age as a dichotomous variable, vadadustat was not associated with a clinically meaningful increase in cardiovascular risk compared to darbepoetin alfa in
U.S. patients treated to a target Hb range of 10 to 11 g/dL, with aMACE HR of 1.06 (0.87, 1.29). This was confirmed using age as a continuous variable as shown below.
- The incidence of treatment emergent adverse events during the
ESA -untreated patients (Correction) study in the vadadustat-treated patients was 90.9%, and 91.6% in darbepoetin alfa-treated patients. During the study, the most common treatment emergent adverse events reported in vadadustat/darbepoetin alfa-treated patients were end-stage renal disease (34.7%/ 35.2%), hypertension (17.7%/ 22.1.%), hyperkalemia (12.3.%/ 15.6%), urinary tract infection (12.9%/ 12.0%), diarrhea (13.9%/ 10.0%), peripheral oedema (12.5%/ 10.5%), fall (9.6%/ 10%) and nausea (10%/ 8.2%). Serious treatment emergent adverse events were 65.3% for vadadustat-treated patients and 64.5% for darbepoetin alfa-treated patients. The incidence of treatment emergent adverse events during theESA -treated patients (Conversion) study in vadadustat treated patients was 89.1% and 87.7% in darbepoetin alfa-treated patients. During the study, the most common treatment emergent adverse events reported in vadadustat/darbepoetin alfa-treated patients were end-stage renal disease (27.5%/ 28.4%), hypertension (14.4%/ 14.8%), urinary tract infection (12.2%/ 14.5%), diarrhea (13.8.%/ 8.8.%), peripheral oedema (9.9%/ 10.1%) and pneumonia (10.0%/ 9.7%). Serious treatment emergent adverse events were 58.5% for vadadustat-treated patients and 56.6% for darbepoetin alfa-treated patients.
"The newly presented pre-specified analyses of PRO2TECT showed that vadadustat had no clinically meaningful increase in cardiovascular risk in
Akebia plans to submit to the
Investor Briefing Webcast
Akebia management will host an investor briefing webcast with Dr.
About the PRO2TECT Global Phase 3 Program of Vadadustat
Akebia's global PRO2TECT program included two separate Phase 3 studies of
About Anemia due to Chronic Kidney Disease (CKD)
Anemia is a condition in which a person lacks enough healthy red blood cells to carry adequate oxygen to the body's tissues. It commonly occurs in people with CKD because their kidneys do not produce enough erythropoietin (EPO), a hormone that helps regulate production of red blood cells. Anemia due to CKD can have a profound impact on a person's quality of life as it can cause fatigue, dizziness, shortness of breath and cognitive dysfunction. Left untreated, anemia leads to deterioration in health and is associated with increased morbidity and mortality in people with CKD.
About Vadadustat
Vadadustat is an oral hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor designed to mimic the physiologic effect of altitude on oxygen availability. At higher altitudes, the body responds to lower oxygen availability with stabilization of hypoxia-inducible factor, which can lead to increased red blood cell production and improved oxygen delivery to tissues. Vadadustat is in global Phase 3 development for the treatment of anemia due to CKD and is not approved by the
About
Forward Looking Statements
Statements in this press release regarding Akebia's strategy, plans, prospects, expectations, beliefs, intentions and goals are forward-looking statements within the meaning of the
Investor Contact
Ir@akebia.com
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/akebia-presents-results-from-its-pro2tect-global-phase-3-program-of-vadadustat-for-the-treatment-of-anemia-due-to-chronic-kidney-disease-in-adult-patients-not-on-dialysis-during-late-breaking-session-at-american-society-of-nephrol-301158891.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/akebia-presents-results-from-its-pro2tect-global-phase-3-program-of-vadadustat-for-the-treatment-of-anemia-due-to-chronic-kidney-disease-in-adult-patients-not-on-dialysis-during-late-breaking-session-at-american-society-of-nephrol-301158891.html
SOURCE


Akebia Therapeutics, Inc.
245 First Street, Suite 1400
Cambridge, MA 02142
+1 617.871.2098 phone
+1 617.871.2099 fax