UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_____________________
FORM 8-K
_____________________
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(D)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): June 7, 2023
_____________________
(Exact name of registrant as specified in its charter)
_____________________
| (State or other jurisdiction of incorporation) | (Commission File Number) | (IRS Employer Identification No.) | ||||||||||||
| (Address of principal executive offices) | (Zip Code) | |||||||
Registrant’s telephone number, including area code: (617 ) 871-2098
N/A
(Former name or former address, if changed since last report)
_____________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | ||||||||
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | ||||||||
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | ||||||||
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) | ||||||||
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading symbol(s) | Name of each exchange on which registered | ||||||||||||
The | ||||||||||||||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
| Emerging growth company | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01. Regulation FD Disclosure.
John P. Butler, President and Chief Executive Officer of Akebia Therapeutics, Inc. (the “Company”), plans to present the information in the presentation attached hereto as Exhibit 99.1 (the “Presentation”) at the Jefferies Healthcare Conference on Wednesday, June 7, 2023 at 8:30 a.m. ET. Spokespersons of the Company also plan to present the information in the Presentation at various meetings beginning on June 7, 2023, including investor and analyst meetings. A copy of the Presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information in this Item 7.01 of this Current Report on Form 8-K and Exhibit 99.1 hereto shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities under that Section. The information contained in this Item 7.01 and Exhibit 99.1 hereto shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission (the “SEC”) made by the Company under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
By providing the information in Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1 hereto, the Company is not making an admission as to the materiality of any information herein. The information contained in this Current Report on Form 8-K is intended to be considered in the context of more complete information included in the Company’s filings with the SEC and other public announcements that the Company has made and may make from time to time by press release or otherwise. The Company undertakes no duty or obligation to update or revise the information contained in this Current Report on Form 8-K, although it may do so from time to time as its management believes is appropriate. Any such updating may be made through the filing of other reports or documents with the SEC, through press releases or through other public disclosures.
| Item 9.01. | Financial Statements and Exhibits. | ||||
(d) Exhibits
| Exhibit No. | Description | |||||||
| 99.1 | ||||||||
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |||||||
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| AKEBIA THERAPEUTICS, INC. | ||||||||
| Date: June 7, 2023 | By: | /s/ John P. Butler | ||||||
| Name: John P. Butler | ||||||||
| Title: President and Chief Executive Officer | ||||||||

Commercial Depth. Operational Excellence. A Commitment to Advance Innovation to Address Unmet Needs. John P. Butler, CEO June 2023 Exhibit 99.1

Statements in this presentation regarding Akebia Therapeutics, Inc.’s (“Akebia’s”) strategy, plans, prospects, expectations, beliefs, intentions and goals are forward- looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, as amended, and include, but are not limited to, statements regarding: Akebia's plans, strategies and prospects for its business, including with respect to Akebia's plans to request a Type A meeting and then resubmit its NDA for vadadustat, including the timing thereof and data to be included therein; Akebia's expectations on the timing of review of its NDA once resubmitted; Akebia's plans and expectations with respect to commercializing Vafseo in Europe, including the timing thereof; statements regarding the beliefs about the benefits that vadadustat could provide to patients; Akebia's expectations on the timing for certain regulatory decisions for vadadustat by the FDA and regulatory authorities in Switzerland and Australia; Akebia's future plans with respect to its strategic growth and operating plans; Akebia's revenue guidance for Auryxia in 2023 and assumptions related thereto; Akebia's plans with respect to vadadustat as a treatment of anemia due to chronic kidney disease in patients on dialysis; Akebia's goals, objectives and expectations with respect to its operating plan, expenses, cash resources and sources of funding for its cash runway, including its belief that its existing cash resources and revenues from Auryxia will be sufficient to fund its current operating plan for at least the next twelve months; and the potential therapeutic applications of the HIF pathway. The terms “believe,” “plan,” “potential,” “estimate,” “expect,” “future,” “advance,” “will,” “continue,” derivatives of these words, and similar references are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Actual results, performance or experience may differ materially from those expressed or implied by any forward-looking statement as a result of various risks, uncertainties and other factors, including, but not limited to, risks associated with: the potential demand and market potential and acceptance of, as well as coverage and reimbursement related to, Auryxia, including estimates regarding the potential market opportunity; Akebia's ability to implement cost avoidance measures and reduce overhead costs, including its ability to reduce operating expenses; decisions made by health authorities, such as the FDA and regulatory authorities in Switzerland and Australia, with respect to regulatory filings; the potential therapeutic benefits, safety profile, and effectiveness of vadadustat; manufacturing, supply chain and quality matters and any recalls, write-downs, impairments or other related consequences or potential consequences; the risk that future clinical trials of product candidates may be unsuccessful, including that vadadustat may not be found to be an effective treatment for ARDS; Akebia’s intellectual property position, including its ability to obtain, maintain and enforce patent and other intellectual property protection for Akebia’s commercial product, Auryxia, vadadustat and any other product candidates; and early termination of any of Akebia's collaborations. Other risks and uncertainties include those identified under the heading "Risk Factors" in Akebia's Quarterly Report on Form 10-Q for the quarter ended March 31, 2023, and other filings that Akebia may make with the U.S. Securities and Exchange Commission in the future. These forward-looking statements (except as otherwise noted) speak only as of the date of this presentation, and, except as required by law, Akebia does not undertake, and specifically disclaims, any obligation to update any forward-looking statements contained in this presentation. Akebia Therapeutics®, Auryxia® and VafseoTM are registered trademarks of Akebia Therapeutics, Inc. and its affiliates.

Forward toward Our Purpose to Better the Lives of People Impacted By Kidney Disease

Marked progress in past four months: Approval in the EU and United Kingdom European Commission and Medicines and Healthcare products Regulatory Agency approved Vafseo® (vadadustat) for the treatment of symptomatic anemia associated with chronic kidney disease in adults on chronic maintenance dialysis Clear Path from FDA to Resubmit Vadadustat NDA Office of New Drugs provided guidance to request Type A meeting, provided conclusions on issues identified in the CRL, and outlined information to be included in the resubmission, which did not include the generation of additional clinical data. Selected European Partner Equipped to Launch Vafseo in Europe Medice granted exclusive license to market and to sell Vafseo in European Economic Area, U.K., Switzerland and Australia with launch expected in the EU later this year VADADUSTAT Oral HIF-prolyl hydroxylase inhibitor treatment option for patients with anemia due to CKD on dialysis
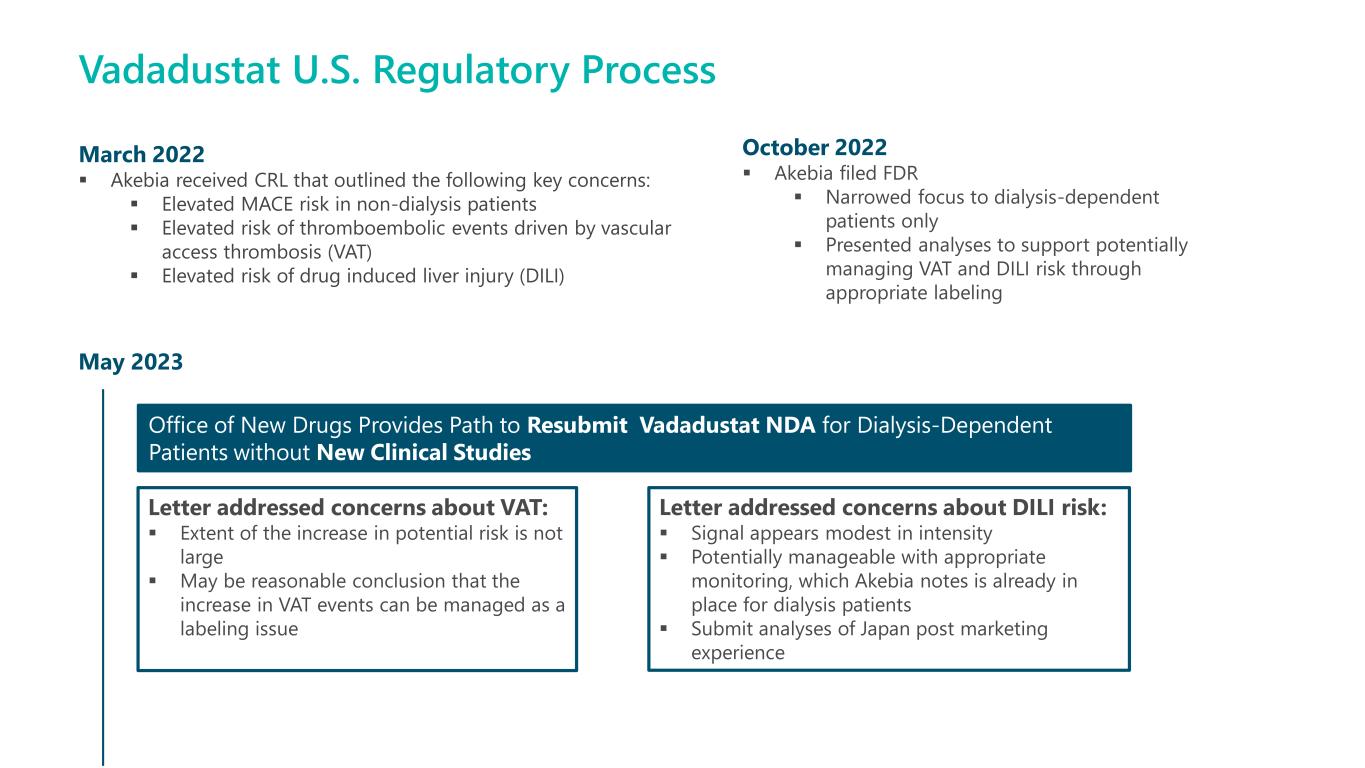
May 2023 Vadadustat U.S. Regulatory Process March 2022 ▪ Akebia received CRL that outlined the following key concerns: ▪ Elevated MACE risk in non-dialysis patients ▪ Elevated risk of thromboembolic events driven by vascular access thrombosis (VAT) ▪ Elevated risk of drug induced liver injury (DILI) October 2022 ▪ Akebia filed FDR ▪ Narrowed focus to dialysis-dependent patients only ▪ Presented analyses to support potentially managing VAT and DILI risk through appropriate labeling Office of New Drugs Provides Path to Resubmit Vadadustat NDA for Dialysis-Dependent Patients without New Clinical Studies Letter addressed concerns about VAT: ▪ Extent of the increase in potential risk is not large ▪ May be reasonable conclusion that the increase in VAT events can be managed as a labeling issue Letter addressed concerns about DILI risk: ▪ Signal appears modest in intensity ▪ Potentially manageable with appropriate monitoring, which Akebia notes is already in place for dialysis patients ▪ Submit analyses of Japan post marketing experience

Type A Meeting with FDA Resubmit NDA for vadadustat for patients on dialysis PDUFA Potential Approval TDAPA Designation* Commercial Launch* June 2024 Prepare Briefing Book, Request Receive minutes Notified of acceptance January 2024 May 2023 Anticipate 6-month review File for TDAPA* January 2025 Product in market* * If approved Illustrative Timeline

Market Opportunity + Launch Readiness ▪ Anemia due to CKD is well recognized, but not always well controlled ▪ Despite available options, a percentage of patients still do not achieve Hb levels within the target range Approximately 88% of 550,000 patients on dialysis are treated with an ESA Akebia is well positioned to maximize value of Vafseo U.S. launch upon approval ▪ Minimal incremental spend to support resubmission of NDA and, if approved, execute successful launch ▪ Existing internal commercial organization with renal expertise ▪ Anticipated Vafseo revenue stream in early 2025 if approved ▪ Regained U.S. rights from former U.S./European partner ▪ Collaboration with CSL Vifor leverages their exclusive distribution into certain dialysis organizations, representing 60% of dialysis market, and Akebia would retain 2/3 revenue Proportion of economics on potential Vafseo U.S. revenue is double what was expected in March 2022 due to termination of collaboration agreement

Global Support for VAFSEO ▪ Vafseo approved in 33 countries including EU and U.K. ▪ Review underway in Switzerland, Australia and Taiwan with responses expected in 2023 ▪ Planning to explore review processes in Canada, China and LATAM Regulatory Status Market Potential ▪ At least 325,000 dialysis patients across Europe are currently treated for anemia due to CKD ▪ Akebia is eligible for up to $100 million in commercial milestone payments, and tiered royalties up to 30% of net sales in dialysis under Medice agreement ▪ Germany-based pharmaceutical company with extensive expertise in nephrology, dialysis and understanding of country-by-country marketing and distribution nuances ▪ Exclusive license agreement with Medice granting rights to market and sell Vafseo (vadadustat) in European Economic Area, U.K., Switzerland and Australia.

Revenue + Cash Management ▪ $177M net product revenue in FY2022 ▪ Reported as of May 8, 2023 revenue guidance of $175-$180M Ongoing financial discipline and anticipated cash to fund operating plan for 12+ months provide foundation to maximize value for Vafseo and fund innovation Revenue to Fund Current Operations Operating Expenses* Full Year 2020-2022 $376M 20222020 $326M $270M 2021 -17% Secured Debt 2020-2022 ▪ Reduced end of year debt balance from $100M in 2021 to $67M 2022 ▪ Debt balance at end of Q1 2023 was $51M

Angiogenesis Iron homeostasis Energy metabolism pH regulation EPO production Cell cycle and growth HIF pathway1-4 Utilizing Akebia’s investigations into the HIF mechanism of action in anemia due to CKD to other hypoxic conditions, such as acute respiratory distress syndrome. The discovery of HIF has laid the foundation to help understand the central role of oxygen sensing in many diseases. 1Maxwell PH, Eckardt KU. Nat Rev Nephrol. 2016;12(3):157-168. 2Kumar H, Choi DK. Mediators Inflamm. 2015;2015:584758. 3Pergola PE, et al. Kidney Int. 2016;90(5):1115-1122. 4Sanghani NS, Haase VH. Adv Chronic Kidney Dis. 2019;26(4):253-266 ▪ Vadadustat lifecycle management: Acute Respiratory Distress Syndrome (ARDS) study with UT Health expected to commence 2H 2023 ▪ High unmet need with ~46% mortality with severe ARDS1 ▪ Progress early HIF research for IND anticipated in 2024 in an acute indication Pipeline + Strategic Growth for Long Term Value Creation

Discovery Phase 1 Phase 2 Phase 3 In Regulatory Review Process Approved In Market Auryxia Hyperphosphatemia Iron Deficiency Anemia United States, Japan and Taiwan Vafseo1 Anemia DD-CKD Anemia NDD-CKD Japan Vafseo Anemia DD-CKD European Union, U.K. vadadustat Anemia DD-CKD United States Switzerland Australia Taiwan vadadustat ARDS3 United States Vadadustat expansion opportunities Exploring multiple indications United States, Europe praliciguat FSGS4 Global Current In-Market Therapies and Development Plans 1Marketed by MTPC 2 Taiwan 5Clinical study conducted by UTHealth Houston 6Further CMC development for clinical trial materials ongoing

Milestones since January 2023 ✓ Vadadustat approved in 33 countries ✓ Agreement with Medice to commercialize Vafseo in Europe, U.K. ✓ Clear path forward for resubmission of vadadustat NDA in U.S. ✓ Continued Auryxia revenue stream ✓ Noted operational cost savings Anticipated Catalysts through December 2023 ❑ Complete vadadustat Type A meeting ❑ Resubmit vadadustat NDA ❑ NDA acceptance by FDA ❑ Support Vafseo launch in Europe ❑ Achieve Auryxia net product revenue guidance ❑ Enroll first patient in vadadustat ARDS study ❑ Publish FOCUS data exploring alternate dosing of vadadustat in dialysis patients

